Research That Begins and Ends With Patients: The Promise of "Reverse Translation"
Akira Sawa, M.D., Ph.D.
Professor, Johns Hopkins School of Medicine and Bloomberg School of Public Health
Director, Johns Hopkins Schizophrenia Center
BBRF Scientific Council Member
2011 BBRF Distinguished Investigator
2004, 2002 BBRF Young Investigator
Starting with problems faced by psychiatric patients, Dr. Akira Sawa conducts research in the lab that seeks to bring solutions back to the clinic.
While studying for his M.D. degree, Akira Sawa, like his peers, thought carefully about what kind of medicine he wanted to practice. For a while, during a clerkship in surgery, he was captivated by the idea of becoming a surgeon. Observing with delight the art of experienced doctors performing liver transplants, he found the results to be “almost magical.” Indeed, the high success rate of such transplants by the late 1980s did seem like magic, unless you understood, as Dr. Sawa notes, that such results were dramatically facilitated by the development of immunosuppressing drugs. These are medicines that greatly minimize the chance that the transplant recipient’s body will reject the donor’s organ—which the immune system recognizes, correctly, as “foreign tissue,” and tries to attack.
Such innovations impressed young Dr. Sawa. Recognizing the significance of mechanism- driven medical research, his interest in surgery waned. “I began to think about what areas of medicine needed the most innovation” he remembers. “Which discipline was most underdeveloped? Unfortunately, it seemed to be psychiatry.” He thought that if he went into psychiatry, “I could contribute to its daily practice through patient-based medical research and scientific innovation.”
Today a figure of international renown, Dr. Sawa holds professorships at the Johns Hopkins School of Medicine and Bloomberg School of Public Health, in several fields: Psychiatry and Behavioral Sciences, as well as in Mental Health; Genetic Medicine; Pharmacology and Molecular Sciences; Biomedical Engineering; and Neuroscience. He also is Director of the Johns Hopkins Schizophrenia Center, where his direct involvement with patients continues, often in connection with his supervision of medical interns, along with his many research and administrative commitments.
The central idea that drives Dr. Sawa is closely related to that with which he began: he strives very consciously to connect what doctors observe of their patients in the clinic to the research projects they choose to undertake in the laboratory. Over the decades of his career, Dr. Sawa, who has received three BBRF research grants and is a member of BBRF’s Scientific Council, has come to dedicate his energies to an approach called “reverse translation.” To establish this approach, he has focused, especially over the past decade, in building clinical cohorts composed of patients with psychotic disorders, from whom he and colleagues collect not only clinical but also brain imaging and molecular and cellular data from biopsied tissues.
“We always start from the clinic—from our patients,” he explains. “What we want to discover is how our clinical observations can be explained in terms of biological mechanisms, or how they can be understood in a biological context.” The aim is to then translate this newly obtained biological insight back to the clinic—in the form of biomarkers to aid diagnosis, or new targets for better treatments, or even those treatments themselves. It’s called “reverse translation” to distinguish it from “translational research” that proceeds from observations and discoveries made initially in the laboratory, which may or may not have a direct relationship with observations made in patients (in this case, those with psychiatric illness).
Both traditional translational research and reverse translation are vitally important. Both share the ultimate aim of improving the lives of patients, and Dr. Sawa would be the last to suggest that reverse translation is intrinsically more important. What he does strive to show, however, is that the approach that begins with patients and leads via the laboratory back to patients can be particularly powerful in advancing doctors’ ability to practice precision medicine—the idea of tailoring treatments to individual patients, or sub-groups of patients, who might share a broad diagnosis like schizophrenia or depression but for whom the optimal treatment may differ.
The best way to explain the promise of reverse translation is through examples, of which there are several in the recent history of Dr. Sawa’s lab. The three that we will summarize in this story might be thought of as three stories that begin with observations in psychiatric patients that posed a mystery to doctors and researchers— phenomena that were clearly occurring in patients but could not be accounted for based on the current understanding of human biology. These mysteries in each instance prompted experiments in the laboratory, which in turn have culminated in discoveries that may have translational value for precision medicine.
MYSTERY 1: How to explain conflicting signals from the immune system in schizophrenia?
In 2022 Dr. Sawa and his team published a study in the journal Nature that addressed a potentially important clinical inconsistency. For years, they and others had been interested in whether or how the body’s immune response— and the inflammation it can cause— plays a role in schizophrenia.
It was logical to routinely collect and analyze cerebrospinal fluid (CSF) from patients—a clear liquid bathing the brain and spinal cord that helps to keep the central nervous system healthy, in part by providing it with the protection of the immune system. It had been well established that in the CSF of schizophrenia patients, levels of tiny signaling proteins called pro- inflammatory cytokines were elevated, compared with levels in the CSF of healthy people. Why would this be the case?
Since these cytokines are released when the immune system has been activated, this observation supported the idea that immune activation might play a role in schizophrenia pathology. The problem with this idea was conflicting evidence also coming from schizophrenia patients. Imaging studies with patients with pro-inflammatory cytokine elevation in their CSF offered evidence of no consistent increase in the activation of immune cells in the brain.
The team led by Dr. Sawa observed this discrepancy in his clinical cohorts, and other groups acknowledged it. The inconsistency is potentially important because many studies have shown that the primary immune cells of the brain, called microglia, can affect brain circuit connectivity and the way neurons function. Their main function is to protect the brain, but it had been proposed that microglia might not be functioning properly under conditions of immune challenge spurred by environmental stress. Perhaps, the team reasoned, this would help to explain schizophrenia pathology in at least some fraction of cases.
Dr. Sawa’s team hoped to shed light on the immune activation mystery in schizophrenia patients by going into the lab and performing a series of sophisticated experiments, some in test tubes but most of them in mice, examining what happens to young microglia in the fetal brain after the mother, during pregnancy, has been subjected to stress. “Maternal immune activation” or MIA, during pregnancy, was a promising way to study schizophrenia pathology given that prenatal immune stress and perinatal implications are regarded major risk factors for the illness. Dr. Sawa wanted to know: what happened to microglia in the offspring of mouse mothers that had been stressed during pregnancy?
The team compared the responsiveness of microglia from adult MIA offspring with that from offspring of mice that had not experienced stress during pregnancy. They could see that the activation patterns of many genes in the two sets of microglia differed; 76% of genes that had different activation patterns in adult MIA offspring were downregulated—their expression levels were lower. This was traced, in other experiments, to alterations in a process called epigenetic regulation that helps maintain expression patterns—when and how often particular genes are activated in the cell nucleus (in this case, in microglial cells).
Importantly, many of the “downregulated” genes were recognized to be involved in immune response pathways, whose function was also found, in subsequent experiments, to be altered, in the same animals. Consistent with the epigenetic finding, the team concluded that the alteration in immune response pathways was acquired in response to MIA before birth—it was a result of the activation of the maternal immune system—and was maintained postnatally, into adulthood after these animals were born.
Reflecting molecular changes, these results appeared to relate back to the original mystery in schizophrenia patients that set all of this research in motion. Dr. Sawa’s team found that “blunted” activity of microglia in the fetal period (following maternal stress) corresponded, once the same animals matured, into bunted microglial reactivity to immune challenges in adulthood. The experiments provided an example of how an injury or insult suffered by individuals prior to birth affected brain biology in a lasting way.
How did this relate to schizophrenia patients and the puzzling data Dr. Sawa’s team had collected from them? Cytokines or other proteins carrying the signal of immune threat may be generated in adults with schizophrenia, but in the brain, their microglia seem unable to respond properly—as suggested by the brain imaging data the team had previously collected. This lack of cellular response explains, at least in principle, how a vulnerability or pathology incurred in the fetal period might play out many years later, after birth—perhaps in people, as the Sawa lab’s experiments showed that it can in mice.
In a final round of experiments, the team administered a drug to pregnant mice that had been subjected to immune-activating stress. The drug, as the team intended, had the effect of deleting the population of microglia in their fetuses that had been impacted by the mother’s stress. Also as expected, a natural process ensued: the fetal brain was repopulated with microglia. But these new cells had not been exposed to the mother’s earlier immune challenge and did not show blunted reactivity. The same animals, after birth, grew into adults showing none of the immune activation deficits seen in the adults whose microglia and immune pathways had been impaired prenatally by maternal stress.
This research has not yet led all the way back to the clinic, although it may help us understand what happens to the immune response in the brain—and why—in at least some schizophrenia patients. The team, says Dr. Sawa, is extending this line of research by investigating, among other things, how the timing of maternal stress during pregnancy impacts microglia prior to birth and also once the animals reach adulthood, as well as how such changes affect behavior. Among the translational possibilities, this research might lead to efforts aimed at modifying the emerging fetal immune response, a strategy, if it proves feasible, that could conceivably prevent pathology that culminates years later in schizophrenia or perhaps other psychiatric illnesses.
MYSTERY 2: How does early- life stress increase risk for postpartum depression?

A second set of experiments that exemplify the Sawa team’s “reverse- translation” approach also concerns how events that occur early in life—in this case, adolescence—can result in important threats to mental health many years later.
The team’s point of departure was epidemiological evidence indicating that girls who experience significant stress during adolescence appear to be at elevated risk for suffering postpartum depression (PPD) after giving birth. Dr. Sawa, in collaboration with his colleagues with an expertise in postpartum depression, has confirmed this observation. But why would it be so? How is an environmental exposure translated into a biological vulnerability—and one that may not manifest for many years? It’s a grand- challenge question that neuroscientists and psychiatrists have asked for many years about this and other early-life insults to the developing brain.
PPD is defined as a major depressive episode affecting women in the period following childbirth. It is the most common complication of childbirth, affecting approximately 10% to 15% of women who give birth in the United States. An estimated 5% to 10% of women who experience PPD have a severe form of the disorder, posing a direct threat to the life of the mother, and, of course, the welfare of her newborn. Both severe and less severe forms of PPD have been linked with abrupt changes in hormone levels after childbirth, and can be addressed today with rapid-acting medicines (brexanolone and zuranolone) whose development has been influenced by research performed by BBRF grantees.
Genetic variations are likely involved in elevated individual risk for PPD, but environmental exposures to trauma, stress, and other factors are also probably involved in many instances. For Dr. Sawa, research his team performed culminating in a 2013 paper in Science provided a starting point for new experiments a few years ago examining one potential mechanism involved in connecting early-life exposures into elevated risk for PPD. “In our paper from 2013, we made observations about how stress can be converted into long-term effects,” Dr. Sawa says. “We found how adolescent stress may cause long-term changes in gene expression via epigenetic mechanisms, specifically involving the glucocorticoid system and dysregulation of the HPA axis.”
The HPA axis is a communication system involving the hypothalamus, pituitary, and adrenal glands, which form the body’s main stress response network. This network uses hormones to link perceptions of threat or danger with the body’s physiological reaction to such threats—the stress response.
Glucocorticoids are hormones found in almost every human and animal cell, which, when they bind at glucocorticoid receptors in cells, generate a response that is generally anti-inflammatory. They upregulate, or increase, the activity of immune-suppressing proteins, while downregulating, or decreasing, the activity of immune-stimulating proteins.
Women who have experienced adverse life events are three times more likely to have PPD than women who did not experience any adverse life events, Dr. Sawa and colleagues noted in a 2024 paper in Nature Mental Health carrying forward the 2013 research to specifically address how early exposure to trauma may affect PPD risk much later in life. The team knew that patients with depression who have experienced adverse life events tend to be treatment- resistant. For this reason, they investigated the longitudinal effects— the effects over time—of adolescent stress on the HPA axis and postpartum behaviors in mice and people. In mice they observed that social isolation during adolescence caused prolonged elevation in glucocorticoid levels, and dysregulation of the HPA axis.
“Many studies have reported alterations in plasma hormone levels in [human] patients with PPD and a correlation between hormonal changes and onset of PPD symptoms. Thus, we examined the plasma levels of estradiol, progesterone, prolactin, oxytocin, and corticosterone in our animal model,” the team said in their paper.
Among mice that had given birth, the team found that levels of the stress hormone corticosterone, both in animals that had been stressed and unstressed in adolescence, was increased after late pregnancy and the early postnatal period in comparison to adult mice that were not pregnant and had not given birth. To some extent, this elevation is a normal part of pregnancy and childbirth. Corticosterone levels in previously unstressed mothers did begin to decrease after delivery, but importantly, corticosterone levels in mothers that had been stressed in adolescence were found to be consistently higher than those in unstressed controls, both one week and three weeks postpartum.
To the team, these data suggested that a change in the control of the HPA axis might have occurred in the animals that had experienced early- life stress, and that this resulted in a prolonged elevation of corticosterone levels—an elevation that continued into adulthood and affected some animals in pregnancy and postpartum.
To Dr. Sawa’s team, the mechanism tentatively identified in their model mouse system may help explain “an important subset of patients with PPD who experience adverse life events and resultant changes in the HPA axis.”
Half of patients with PPD are known to be resistant to treatment with SSRI antidepressants (like Zoloft or Prozac) and these treatment-resistant cases are likely to be associated with adverse life events. The team used their adolescent stress paradigm to model such clinically difficult cases. In mouse mothers that had adolescent exposure to stress, the researchers tested the postpartum therapeutic impact, if any, of SSRIs; a brexanolone-like drug; and an FDA- approved drug that blocks glucocorticoid signaling (a glucocorticoid receptor antagonist). After one week, only the drug that inhibited the glucocorticoid receptor showed evidence of reducing PPD-like symptoms in the model mice. For this reason, the team suggested that repurposing glucocorticoid receptor antagonists for some cases of treatment- resistant PPD may be a therapeutic approach worthy of testing more in animals and eventually in people.
“What I really want people to appreciate about this work is about connecting the idea of reverse-translation with precision medicine,” Dr. Sawa says. The idea of a brief course of a glucocorticoid receptor- blocking medicine right after delivery is potentially applicable for a subset of patients. The team regards its approach in this work to be strictly “complementary.” Current medications are very supportive of women post- delivery, but there are still a significant number of patients who don’t respond even to recently approved rapid-acting treatments. That is who this work may specifically address—an example, if the approach works, of precision medicine.
MYSTERY 3: Can shared ‘risk’ genes account for symptoms shared across illnesses?
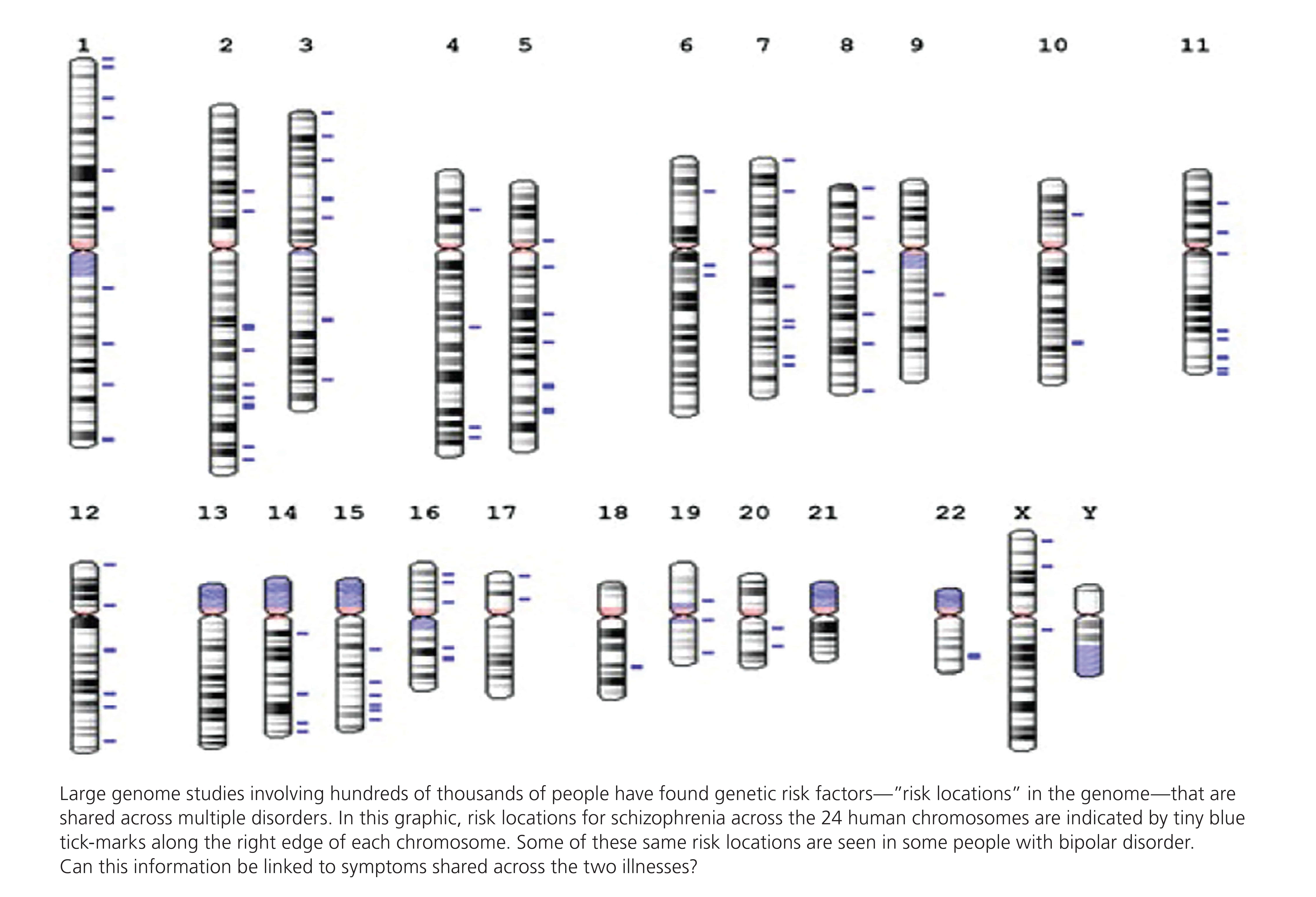
“Remember,” Dr. Sawa stresses, “in what we do, we try always to start from the clinic—from patients. This includes psychiatric genetics. In genetic studies of psychiatric illness, there is always a gap, between genetic risk factors that are identified in these studies and biological mechanisms through which they exert their effects. There is always a gap, and what we are trying to do is fill this gap.”
A third example of reverse-translational research in the Sawa lab concerns the results of genome studies that have found genetic risk factors that are shared in bipolar disorder and schizophrenia. Standard treatment of these illnesses has tended to be based entirely on diagnosis, and treatments for schizophrenia are usually different from those for bipolar disorder (schizophrenia treatments focusing mainly on psychosis symptoms, and bipolar treatments focusing on mood symptoms). But it has long been known that these two illnesses, despite their differences, can share some important and often debilitating symptoms. Some patients with bipolar disorder experience psychosis, which is seen, albeit much more often, in schizophrenia. Both disorders also show neurocognitive impairments in areas including executive functioning and memory.
This sharing of certain symptoms has an intriguing echo in data from genome studies of each illness, which have indicated significant genetic overlaps. That is, in studies comparing genomes of large numbers of individuals, commonly occurring variations in DNA that are consistently seen in people with schizophrenia (compared with people who do not have the illness) are in many instances also seen in people with bipolar disorder, and vice-versa.
Do shared genetic risk factors have anything to do with biological changes that give rise to pathologies in both illnesses, and particularly those which may affect symptoms that are shared across the two conditions?
In 2023, Dr. Sawa and his team published a paper in Neuron presenting what they call “a novel research framework in which human and animal studies are combined to address a disease mechanism that bridges across pathogenesis [causation], pathophysiology [how disease causes functional changes in biology], and clinical manifestations.”
The paper reporting the results of this effort is complex, but its main thrust concerns the impact of one of the common genetic variations linked specifically with both schizophrenia and bipolar disorder. The team showed that this shared DNA variation, in neuronal cells, causes an “upregulation,” i.e., increased levels, of a small RNA molecule (a microRNA) called miR-124.
Dr. Sawa and colleagues discovered an upregulation of miR-124 in neuronal cells biopsied from living patients with schizophrenia and bipolar disorder in the clinical cohorts that Dr. Sawa has established. This upregulation of the microRNA was found to be associated with DNA variations shared across the two illnesses. The next step represents the team’s “reverse-translational” move back into the laboratory, where they created a mouse model to study how the genome variation becomes manifest, mechanistically, in cells, and ultimately is able to have an impact on behavior.
In the model mice created by Dr. Sawa’s team, miR-124 was upregulated in neurons of the medial portion of the prefrontal cortex. The researchers observed that this, in turn, led to an increase in the number of excitatory cellular receptors (called AMPA receptors) in the affected neurons. This proliferation of excitatory receptors perturbed the transmission of signals at synapses, the tiny gaps across which neurons communicate. It was this synaptic dysregulation that the team, in a final step, was able to directly connect (in mouse models) with behavioral deficits seen in both schizophrenia and bipolar disorder— “shared deficits” affecting social behavior and hyperstimulation or hyperactivity. In other words, this research, which began with genome and symptom data from affected patients, led, via experiments in the lab, back to the symptoms that patients actually experience and live with.
The results, Dr. Sawa suggests, can now be integrated into large clinical studies focusing on “revisiting” the train of causation that the team established in their animal model, but this time in the context of actual patients. It might lead, for example, to new biomarker-based diagnostic methods—here, as in the research on PPD, perhaps for a subset of patients—in this case, with the specific vulnerability involving miR-124 and its pathway, who may be diagnosed either with schizophrenia or bipolar disorder. It is also possible that a future treatment involving selective downregulation of AMPA receptors in affected brain areas might be useful in either or both illnesses.
“In the bigger picture,” Dr. Sawa and colleagues concluded in the 2023 Neuron paper, “our new intellectual framework that focuses on the neurobiological mechanism(s) underlying specific behavioral dimension(s)—rather than classical diagnostic categories (e.g., schizophrenia and bipolar disorder) may open new avenues for the discovery of additional key biological pathways” that bridge causation, functional changes in biology, and clinical symptoms in “a wide range of neuropsychiatric conditions, hopefully invigorating drug development pipelines.”
Many people—not least patients and their loved ones—are impatient for new treatments for common and often devastating illnesses such as schizophrenia, bipolar disorder, and depression. The reverse-translation approach being pursued by Dr. Sawa and his team suggests one specific approach that talented researchers have employed to expand the area of what is known in order to provide the eagerly awaited and sorely needed next generation of therapies.
Written By Peter Tarr, Ph.D.
Click here to read the Brain & Behavior Magazine's July 2025 issue



