How Stress-Related Immune Activation May Alter the Brain and Impair Behavior
Scott J. Russo, Ph.D.
Leon Levy Director, Brain and Body Research Center,
Department of Neuroscience,
Icahn School of Medicine at Mount Sinai
BBRF Scientific Council Member
2008, 2006 BBRF Young Investigator
In the fall of 2021, BBRF Scientific Council member Scott J. Russo, Ph.D., and colleagues at the Icahn School of Medicine at Mount Sinai launched the Brain and Body Research Center at that institution. It is composed of researchers and clinicians from diverse specialties, from neuroscience and neurology to cardiology, gastroenterology, dermatology, and immunology, who, says Dr. Russo, “are pioneering a holistic approach to revealing the intricate connections between the brain and body that drive health and disease.”
Central in this effort, which Dr. Russo directs, is to “decode the brain’s conversations” with other organ systems, including the heart, gut, and skin. “We are trying to understand how the brain and peripheral organ systems interact,” he explains, “and, importantly, to understand why there are so many co-morbidities between mental illnesses, neurological conditions, and systemic organ diseases.”
This past year, Dr. Russo, who received Young Investigator grants from BBRF in 2008 and 2006, joined with colleagues including Flurin Cathomas, M.D., a 2020 BBRF Young Investigator; Eric J. Nestler, M.D. Ph.D., BBRF Scientific Council member, prize-winner and past grantee; and six other BBRF grantees, to report in the journal Nature on one specific way in which stress and activation of the immune system in the body may contribute to changes in social behavior—one of the symptoms of depression.
As will be explained later in this story, they demonstrated how stress can cause pro-inflammatory immune cells that are manufactured outside the brain—in the body’s “periphery”—to invade the brain and cause changes that appear to have an adverse impact on behavior.
Tracing potentially causal relationships like this is at the heart of why Dr. Russo and colleagues have formed their new research center. It is virtually a matter of common sense, today, to assume that the brain and body are in various ways “connected.” The critical questions have to do with explaining how they are connected, via which biological mechanisms, and how disturbances in either brain or body—or both—can be understood together (“holistically”). To put this another way: how can highly specific chains of causation be established through research—chains that illuminate potential targets for therapies to treat both brain and bodily illnesses that would not be possible to conceive absent sophisticated understanding of mutual brain-body interactions?
“We’ve known for quite some time that brain-body connections are there,” Dr. Russo says, “and we hypothesized about what they were doing, but in the past we never really had the proper tools to test causality.” But, he adds, “we do have the tools now.” He cites optogenetics (a technology co-developed by BBRF Scientific Council member and past grantee Karl Deisseroth, M.D., Ph.D., which enables researchers to experimentally control neuronal cell firing in animals with beams of colored laser light); imaging tools that enable researchers to map and observe the functioning of neural circuits throughout the brain and body; and tools of immunology, which are enabling them to see how immune cells and nerve cells interact at brain- body interfaces.
STRESS, INFLAMMATION, DEPRESSION
For years there has been much discussion about the relationship of stress to major depression, and the relation of both to inflammation. This has led to some obvious questions: Is it possible that someone with major depression goes on to develop bodily inflammation— thus changing risk for other bodily diseases? What about the reverse: can inflammation in the body somehow cause changes in the brain that give rise to depression? In the same vein: If one is under acute or chronic stress, can that cause depression or raise risk for it? What mechanisms are involved?
As Dr. Russo points out, before proper investigative tools were developed, it had been assumed by neuroscientists and physicians who treat organs of the body other than the brain that having a serious non-brain illness, such as severe heart disease, can “make” a person depressed simply by changing the manner in which one goes about daily life. “Your life is negatively impacted by having heart disease, and it made sense to assume you get depressed because you have to deal with this chronic illness that makes life difficult,” Dr. Russo says. He and others began to think more deeply about this some years ago when a researcher injected an immune system molecule (which had potentially therapeutic pro-inflammatory effects) into patients with hepatitis C. About a third of them soon developed diagnosable depression. To Dr. Russo, this data was suggesting the possibility of causality—that an inflammatory molecule (normally generated by the immune system but in this experiment introduced artificially), when delivered throughout the system via the bloodstream, could help cause behavioral changes such as those seen in depression.
Many were unconvinced. It wasn’t clear that the immune system molecule introduced into these patients was the direct cause of the depression that some reported. Perhaps more powerful was an objection based on biology. We have a protective layer in the brain called the blood-brain barrier that shields us from toxins, viruses, as well as the pro-inflammatory immune molecules that circulate in the blood. An inflammatory immune molecule introduced into the bloodstream, it was once assumed, should not be able to penetrate the blood-brain barrier. (The brain has its own defense system composed of cell types only seen in the brain—e.g., microglia and astrocytes.)
But what if this protective barrier was modified by stress or by other factors? “That’s where my lab came into the picture,” Dr. Russo explains. He and colleagues made good use of animal models—rodents (with brains very similar to the human brain due to commonalities in evolution) that can be the subjects of experiments in the lab in which, for example, inflammatory molecules are introduced during stress, while the brain is closely monitored.
“Our brain clearly senses stress,” Dr. Russo notes, “and work that Flurin [Cathomas] in our lab was involved in, in collaboration with the lab of Dr. Fil Swirski, Director of the Cardiovascular Research Institute at Mount Sinai, showed that the brain areas that sense stress also send neuronal projections out to the periphery of the body. For example, to the bone marrow, where leukocytes (white blood cells) and inflammatory molecules are manufactured.”
Dr. Swirski and others showed that in the presence of stress, activation of this pathway from the brain via the nervous system to the bone marrow causes stem cells in the marrow to be activated and to generate a class of cells called inflammatory monocytes, which then get released into the bloodstream.
It is important before we trace what happens next, to understand that the operation of the body’s innate immune system is being described here. Under conditions of stress, but also, a wide range of threats from bodily invaders like viruses, this first-line defense of the immune system is triggered. It happens many times every day of our lives, and helps keep most of us healthy most of the time. A challenge sets the immune system into action, and it responds by sending immune cells into the blood and thus to every point in the body’s “periphery.” Certain activated immune cells cause inflammation by design; that helps explain why they are able to kill invaders and other threats. It’s why your finger gets hot and swells up when a bad cut gets infected. But what if such molecules were active in the brain?
PENETRATING THE BLOOD- BRAIN BARRIER
Inflammation has its place in health. But not only in health. In people experiencing chronic stress, past research has shown that the innate immune system is activated, resulting in the mobilization of immune cells including white blood cells in peripheral organs and blood, as well as the production of tiny proteins called cytokines, which, like pathfinders, are sent out into the body and can trigger inflammation by attracting immune cells to the site of a problem.
One intriguing discovery in psychiatry research has been that a subset of people with stress-related psychiatric disorders, including major depression, display a state of chronic low-grade inflammation. Two phenomena associated with such inflammation are an increase in the affected individual’s pro-inflammatory cytokines in circulation throughout the body, as well as an increase in white blood cell numbers.
Some people appear to be at greater risk for inflammatory damage than others, due to genetics or to life experiences. A healthy child who becomes the target of abuse or is exposed to violence or other trauma may develop an overactive or over- zealous immune system. Might having an overactive immune system (for whatever reason) be a risk factor for illness, including mental illness?
In experiments in recent years by Drs. Russo, Cathomas and others in mice, evidence has been generated suggesting how stress can induce changes to the blood-brain barrier. When modified by stress, the barrier in mice leaks a bit, allowing the entry of circulating proteins into the brain that normally cannot pass through. One region in the brain particularly affected by such invasion following stress, in mice, is the nucleus accumbens (NAc), which is central in the processing of rewards and also in the response to aversive stimuli, and implicated in depression.
Why the NAc? What is distinctive about it that makes it vulnerable to stress-induced local disruption of the blood-brain barrier? “The short answer is we don’t know,” says Dr. Cathomas, “but we do know that when you look at endothelial cells—those are the cells that line the blood-facing side of the barrier—there is a huge heterogeneity.” The diversity of subtypes of cells that make up the barrier contributes to the emerging fact that in different organs, as well as in different regions in the brain, the cell types that make up the barrier often differ from one to the next and likely have different properties.
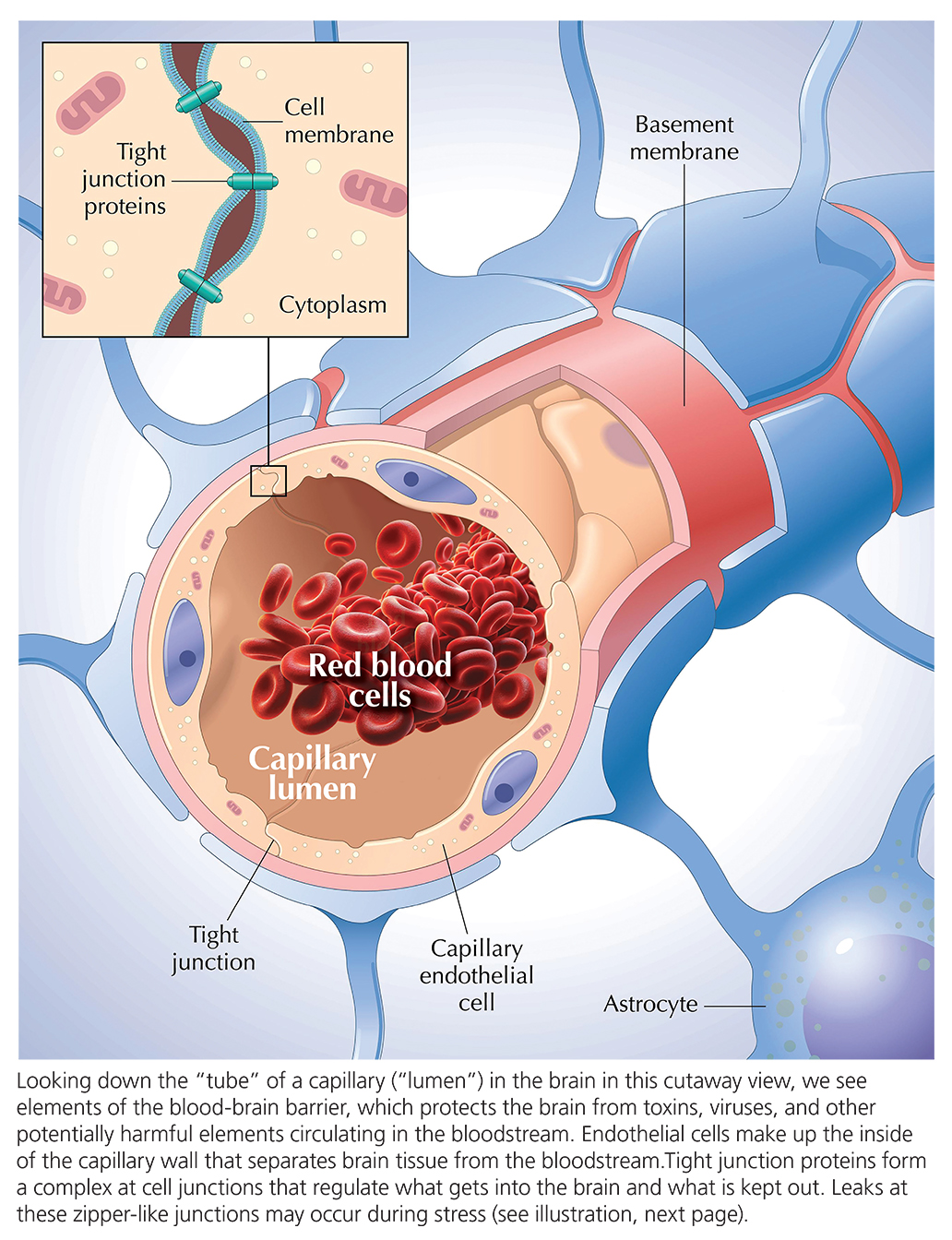
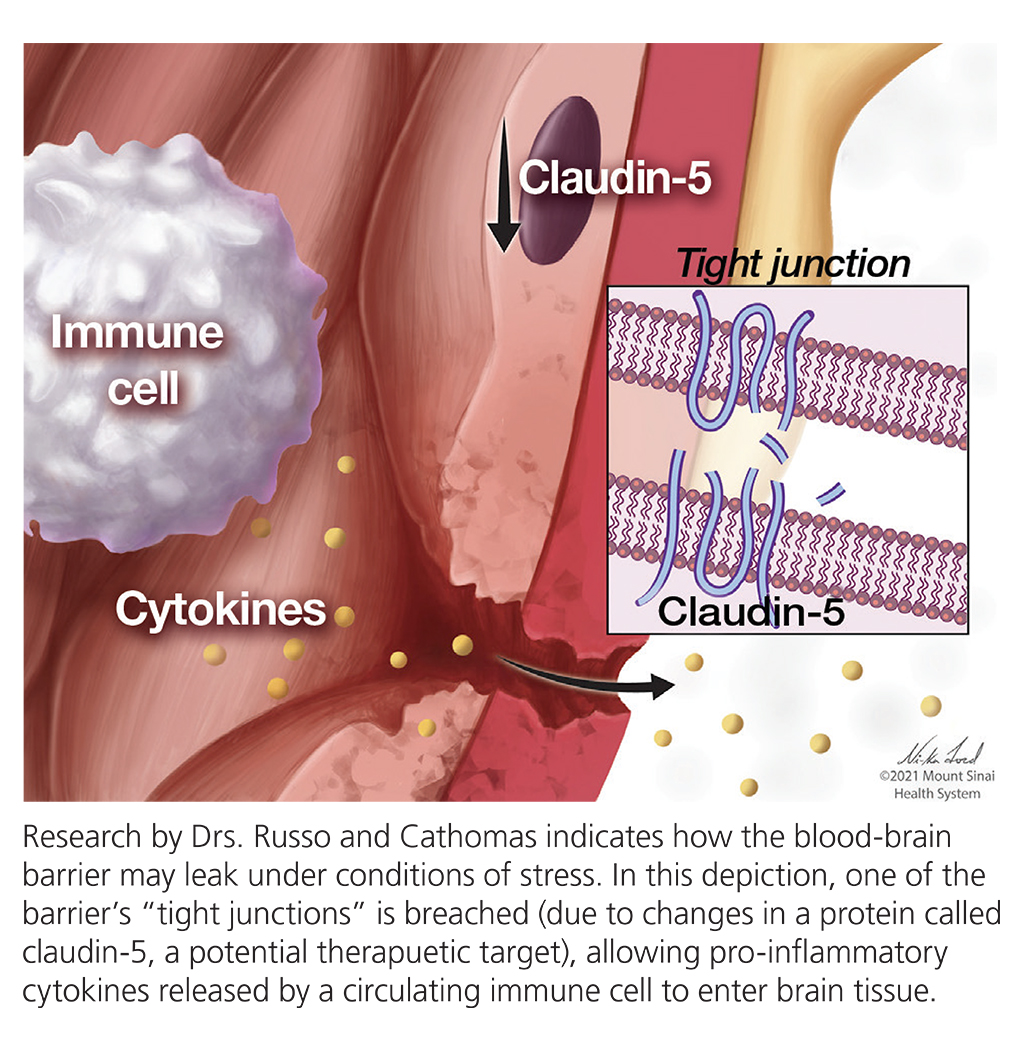
It’s a mistake, say Drs. Cathomas and Russo, to think of the “barrier” as an impervious “Great Wall.” The cells making up the barrier vary from region to region, each with potentially distinct relationships with cells circulating in the blood including immune cells. Thus, under certain conditions of stress, the barrier may behave differently in different bodily areas. The point, says Dr. Russo, is that the blood-brain barrier “is definitely not like a Great Wall. It’s very plastic. Its shape and function undergo changes all the time.” If you are acutely stressed, he suggests, the barrier might open up, transiently. It is possible that the particular configuration of the barrier in NAc renders it particularly plastic when we experience stress.
HOW STRESS CHANGES BIOLOGY—AND BEHAVIOR
In the new experiments Drs. Russo, Cathomas and team reported recently in Nature, they demonstrate a distinct way in which stress promotes interactions of immune cells in the periphery with the brain—an indirect way that they have succeeded in linking with adverse changes in social behavior.
The research uncovered the role in this process of enzymes called MMPs (matrix metalloproteinases) and, in particular, MMP8. This enzyme, like others in the MMP family, has roles in shaping and regulating the space between neurons, called extracellular space (ECS), as well as the extracellular matrix (ECM), which is a dense web-like material that individual neurons extend out into ECS.
“The extracellular matrix is a supporting structure and is really key in many physiological processes,” Dr. Cathomas says. “It’s also a really important structure for the blood-brain barrier,” a key factor in giving structure to this membrane that separates the bloodstream from brain tissue. “It has so many functions and therefore it has to be plastic and is changing all the time.”
Experiments by the team in mice and humans leads them to conclude that MMP8, which is released during chronic social stress by immune cells circulating in the body’s periphery, can invade the brain perhaps due to damage to the blood-brain barrier, and alter the shape of ECS and ECM in the brain’s NAc and possibly other brain areas.
In mouse experiments, such changes were causally linked by the team with changes in behavior—changes (social avoidance, for example) like to those observed when a person experiences chronic social stress.
One potential implication is that it may be possible to develop treatments that target not the brain directly (which is always difficult, in part because of the blood-brain barrier), but rather molecules such as MMP8 in the peripheral immune system that circulate in the bloodstream—a totally novel approach to potentially treat psychiatric illnesses including depression.
The irony, notes Dr. Ruusso, is that remodeling of the extracellular matrix—possibly the culprit in this story of how chronic stress leads to behavioral problems—is not the full picture. “There is another situation in which you definitely want remodeling of the ECS, for example in the hippocampus. You’re trying to form a new memory, and to do so you need to strengthen synapses or add new synapses to change the properties of the cells that are holding those memories. In order for that to happen, the ECS [i.e., the space between neurons] has to open up. I think of this as our brain’s way of opening up windows of plasticity throughout our lives. Unfortunately, in the NAc, when we are under stress this plasticity might just be a bad thing.”
The complex behavior of MMP8 has important implications. Dr. Russo notes that pharmaceutical companies experimented some time back with drugs to target various members of the MMP enzyme family, but since the different members are so structurally similar, the drugs were not specific enough to warrant development. One MMP-targeting drug is being tested in heart disease, but Dr. Russo thinks the better opportunity is to develop extremely specific agents like monoclonal antibodies, which are designed only to “hit” targets with a specific molecular structure.
Other approaches are also possible. But another reflection on the MMP8 discovery involves the realization that it is but one of many factors that influences the shape of the space between cells as well as the shape and permeability of the blood-brain barrier. Future research will seek to identify more factors that have these roles, any of which could potentially be future targets. Drs. Russo and Cathomas have co-authored studies on proteins such as claudins and adhesion molecules which are integral in establishing and influencing the behavior of the blood-brain barrier and hence may have value as therapeutic targets.
Still another issue that arises from the team’s Nature paper concerns the impact that stress-generated immune activity, translated to the brain, has upon behavior. In the team’s mouse experiments, the impact was seen specifically in social behaviors. As Dr. Cathomas points out, mice are extremely social creatures, and their avoidance of social contact after stress-related immune activation is therefore very important.
Dr. Russo notes that data from humans suggests that “disturbances in systemic immunity and inflammation seem to be most associated with anhedonia.” Anhedonia, or the avoidance of or inability to experience or seek out pleasure, is a classic symptom of depression, and is often expressed in terms of avoiding social relationships and human contact. “What Flurin’s data suggests is that the mice in our experiments no longer found social targets rewarding, and that is why they were avoiding them.”
But Dr. Russo thinks that research eventually will reveal immune-sensitive factors that influence behavior in non- social domains. He also is intrigued by thinking of the relationship between immune activation and behavior that may help us better understand some of the behavioral and mood effects of “long COIVD.” Just as stress can cause cells in the periphery of the body to activate immune factors which in turn impact the brain, so too might the “cytokine surge” documented in COVID inflections. His lab is currently studying this.
Perhaps most of all, Dr. Russo is excited about what the recent work in his lab suggests about the co-morbidity of brain and bodily illnesses, a main focus of the Brain and Body Research Center that he directs. “Flurin’s work has shown us that immune system honing signals not only reach the brain, but can also track to cardiovascular plaques”—buildups of fatty cells in the walls of the heart and vasculature that cause heart disease.
“It’s in this sense that we have begun to think of monocytes and other innate immune cells and their products as an anchor for comorbidities. You get stressed out, your bone marrow starts dumping immune cells into the circulation. They go to your brain and cause emotional disturbances. But also, it’s conceivable that if you’re at risk and you have a bad diet and you are overweight and have lots of plaque buildup, it’s possible that inflammatory molecules go to the plaques and perhaps contribute to their rupture, causing a heart attack. This is how we are beginning to think about how brain and body systems interact.”
Written By Peter Tarr, Ph.D.
Click here to read the Brain & Behavior Magazine's February 2025 issue



