Variations in Schizophrenia-Linked Gene Are Linked With Pathology in Prefrontal Cortex, Impairing Cognition
Variations in Schizophrenia-Linked Gene Are Linked With Pathology in Prefrontal Cortex, Impairing Cognition
Several hundred genetic variations have been consistently associated with increased risk for schizophrenia. People who have these DNA variations in their genome have a greater statistical chance of developing the illness—in a few instances, a much greater chance—although precisely how and why DNA variations affect the biology of the brain and contribute to pathology are questions that are just beginning to be understood.
Recently, a research team led by BBRF Scientific Council member Amy F. T. Arnsten, Ph.D., of Yale University, published results in JAMA Psychiatry of an investigation into the linkages between one of the best-validated schizophrenia “risk genes,” called CACNA1C, and processes in the brain that may contribute not only to schizophrenia, but other illnesses including Timothy Syndrome, which causes cardiac and cognitive symptoms in patients, as well as bipolar disorder and Alzheimer’s Disease.
Dr. Arnsten, winner of BBRF’s 2015 Goldman-Rakic Prize for outstanding achievement in cognitive neuroscience, and a 2008 BBRF Distinguished Investigator and 1998 Independent Investigator, has focused her research on the dorsolateral prefrontal cortex (DLPFC), important in higher cognition and abstract reasoning. She has sought to elucidate molecular mechanisms that determine the strength of network connections and cognitive abilities, with the overarching goal of understanding how genetic insults lead to symptoms of mental illness.
The CACNA1C gene, when activated, instructs cells to manufacture a portion (“subunit”) of a tiny nerve-cell pore called the L-type calcium channel (LTCC) Cav1.2. This channel regulates the entry of charged calcium atoms (“calcium ions”) into neurons. A host of slightly different variations of the CACNA1C gene have been identified, some of which reduce calcium entry, and others which increase calcium entry into neurons. Both types of mutations have been associated with impairments in working memory and other cognitive functions of the DLPFC, and with increased risk of mental illnesses.
The question Dr. Arnsten and colleagues, who include two other BBRF Scientific Council members and four other BBRF grantees, sought to answer was: why is this particular calcium channel so important for the proper functioning of the DLPFC and for cognition? They knew they could not search for the answer in mouse models, since rodents do not have a DLPFC, a product of relatively recent evolution and found only in higher primates. The study therefore was performed using postmortem human brain samples and, under strictly regulated and humane conditions, in macaques.
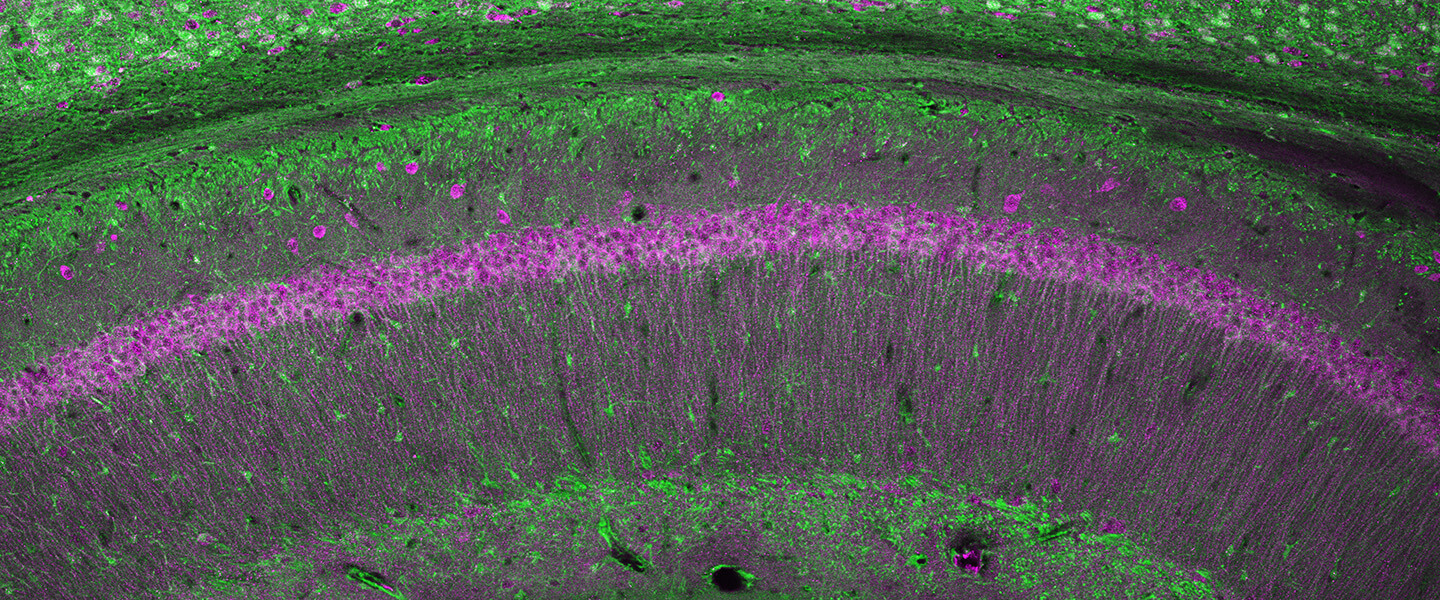
In particular, the team was interested in looking closely at excitatory neurons called pyramidal cells in layer III of the DLPFC. These are the neurons needed to generate working memory and higher cognition. This layer has been shown to be a focus of pathology in schizophrenia in past research, where there are marked reductions in neural connectivity points called spines and dendrites. Layer III is also a focus of pathology in Alzheimer’s disease, where neurons degenerate due to the accumulation of misfolded tau proteins. It has been found that layer III cells in the DLPFC that are most vulnerable to Alzheimer’s pathology express a protein called calbindin that buffers the effects of calcium in the cells. This buffering is important because calcium, when unregulated, can be toxic. Calbindin binds to calcium atoms in a protective manner, but its levels naturally diminish with age and when inflammation is present, leaving neurons more vulnerable to calcium’s toxic effects.
The researchers studied the gene-expression patterns of pyramidal cells in the DLPFC in humans and macaques to learn why those in layer III that expressed the gene that encodes calbindin are so distinctly vulnerable to pathology—and to see if this was related to variations in calcium signaling.
The study generated many important results, chief among them the identification of a constellation of calcium-related signaling proteins in pyramidal cells expressing the gene that encodes calbindin in layer III of the DLPFC—the cells known to be most vulnerable in cognitive disorders. These neurons also expressed high levels of the calcium channel CACNA1C, as well as several other proteins involved in calcium signaling.
The team showed that all these proteins are expressed on the dendritic spines of layer III DLPFC neurons, near the sites where the neurons communicate, positioned to strengthen or weaken network connections. The researchers recorded activity from these DLPFC neurons in macaques, and found that either too little or too much Cav1.2 channel activity impaired neuronal firing needed for working memory. This helps explain why genetic variations that either cause the CACNAC1 gene to gain or lose function have been associated with increased risk of cognitive disorders.
They also found that detrimental Cav1.2 activity is increased by stress, which is an aggravating factor for most mental illnesses. The findings suggest that people with “gain-of-function” mutations in the CACNA1C gene—mutations that add abnormal functionality to the protein—would be particularly vulnerable to stress exposure. This, said the team, “reveals a powerful mechanism by which stress impairs DLPFC cognitive function,” while also supporting the finding that CACNA1C variations “would be harmful to DLPFC function and increase the risk of neuropsychiatric disorders.”
Summing up, the researchers said their results “help explain why genetic variants that either decrease or increase CACNA1C actions would be associated with prefrontal cortex dysfunction and thus increased risk of mental disorders such as schizophrenia.” The results also showed why loss of calbindin from layer III pyramidal neurons with age and/or inflammation would contribute to neuropathology in Alzheimer’s, as the toxic actions of calcium would proceed unchecked.
The study thus provides “a rare example,” they said, of how data about gene expression and genomic variation can be directly linked with dysfunction of a higher cortical circuit, “illuminating how molecular insults give rise to symptoms of cognitive impairment.”
Protecting vulnerable neurons in layer III of the DLPFC would be a helpful strategy to maintain healthy cognitive function, the team suggested. It is still not clear how these cells might be specifically targeted. This is likely to be the subject of future research.
The team included: David A. Lewis, M.D., BBRF Scientific Council, 2008 BBRF Distinguished Investigator, 2005 BBRF Leiber Prize winner; Pasko Rakic, M.D., Ph.D., BBRF Scientific Council, 1995 BBRF Distinguished Investigator; Guillermo Gonzalez-Burgos, Ph.D., 2004, 2000 BBRF Young Investigator; Sabina Berretta, M.D., 2001, 1998 BBRF Young Investigator; Christopher H. van Dyck, M.D., 2000 BBRF Independent Investigator; Min Wang, Ph.D., 2004 BBRF Young Investigator.



